The epidemiology of Bluetongue Virus (BTV) continues to shift throughout Europe, with new outbreaks reported in 2024: BTV-3 in France, Spain, Austria, Italy, and Poland; BTV-12 in the Netherlands; BTV-4 in Austria; and BTV-1 in Spain. In this evolving landscape, effective diagnostic methods are crucial for managing the disease and must be tailored to meet different objectives1.
On one hand, methods that detect the pathogen—such as RT-qPCR or virus isolation—are particularly useful for confirming clinical cases, monitoring the spread of the disease, and checking animals before they are moved. On the other hand, serological techniques like ELISAs and Virus Neutralization Tests (VNT) are mainly used to assess the overall status of a population and evaluate the extent of disease dissemination. Vaccination remains a cornerstone of BTV management in affected countries, and the selection and application of diagnostic tools in this context present several challenges.

Fundamentals of BTV
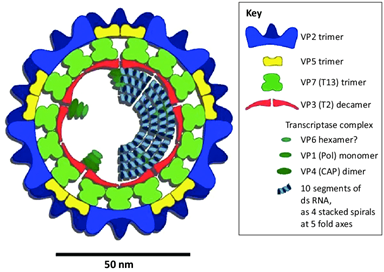
BTV is a member of the Orbivirus genus, which also includes the closely related Epizootic Hemorrhagic Disease Virus (EHDV). There are 36 known serotypes of BTV, with 24 being vector-borne and notifiable. The virus structure comprises:
- 7 Structural Viral Proteins (VP1-VP7)
- 5 Non-Structural Proteins (NS1, NS2, NS3a/3b, NS4)
A schematic representation of the Bluetongue virus is provided in Figure 1. The VP7 core protein is conserved among various serotypes, which means it determines the serogroup, whether BTV or EHDV. Meanwhile, VP2—and to a lesser degree VP5—elicit the production of neutralizing antibodies, defining the virus serotype.
Diagnostic tools for BTV
Several techniques exist to detect antibodies against BTV, including Agar Gel Immunodiffusion (AGID), Viral Neutralization (VNT), and Enzyme-Linked Immunosorbent Assays (ELISAs). ELISA is frequently favored due to its simplicity, objectivity in results (free from operator’s subjective interpretation), and its capacity to handle a large number of samples quickly.
For direct virus detection, methods such as viral isolation and RT-PCR—particularly real-time RT-PCR (RT-qPCR)—are employed, with RT-qPCR being the most commonly used technique today. Many RT-qPCR are described in the literature, in the WOAH manual or commercially available. Assays targeting nucleic acids of Non-Structural Proteins, such as Segment 10 coding for the NS3 protein, can detect any serotype, whereas those aimed at Segment 2 (coding for the VP2 protein) serve as reliable tools for serotyping.
Are Bluetongue ELISAs suitable for post-vaccination monitoring? How should the results be interpreted?
Many BTV vaccines are inactivated and based on
whole viruses. The efficacy of these vaccines hinges on the quality of the
antigen and its dosage. High-quality vaccines prevent clinical signs and
viremia. Depending on the recommended vaccination protocol, one or two doses
may be required. While vaccination induces a strong protective response through
anti-VP2 antibodies, the level of VP7 antibodies produced in naïve animals is
typically low—often falling below the detection threshold of VP7
cELISAs—resulting in negative or only weakly positive outcomes.
How to assess the protection level in vaccinated animals?
The Virus Neutralization Test (VNT) remains the preferred method to confirm that vaccinated animals develop neutralizing, protective antibodies against the VP2 protein for a given serotype. Since VP7 antibodies do not correlate with protection, VP7 serology is not recommended for post-vaccination monitoring or assessing vaccine efficacy. Moreover, VP7 ELISA cannot be used as a DIVA (Differentiating Infected from Vaccinated Animals) test, because repeated vaccinations against different serotypes—or natural infection with another serotype in vaccinated animals—can lead to detectable or even high VP7 antibody titers.
Why is using a VP2-based ELISA scientifically challenging?
European regulations, under the new Animal Health Law, require proof that animals are protected against specific serotypes. Since VNT is technically challenging for this purpose and therefore not applicable, one might consider serotype-specific VP2-based ELISAs as an alternative. However, these assays face significant challenges: the VP2 proteins of different BTV serotypes share substantial sequence homology, leading to considerable cross-reactivity between serotypes. This cross-reactivity, which is already documented in VNT (the reference technique)2,6, is even more pronounced in ELISAs—especially indirect formats. Consequently, VP2-based indirect ELISAs are unable to selectively detect antibodies against a single serotype, whether those antibodies result from vaccination or natural infection. Despite many attempts, no reliable serotype-specific ELISAs have yet been developed.
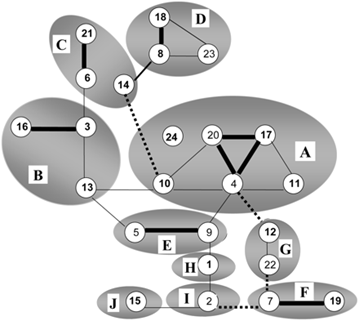
On figure 2, related BTV serotypes based on genome segment 2 expressing serotype-specific immunodominant outer shell protein VP2 are grouped by circles.
Cross-neutralization between BTV serotypes is indicated by lines; strong (thick), some (normal) and weak neutralization (dashed).
Then how to prove protection in animals?
Given the technical difficulties in proving that animals are protected against a specific serotype, the following measures are generally adopted to ensure safe animal movement:
- Proof of Vaccination: Animals must be vaccinated with a vaccine that prevents viremia; in some cases, a negative PCR result is required if animals are moved before full vaccine protection is established.
- Sanitary Measures: These include 15-days insecticide treatment combined with a negative PCR result.
The exact measures may vary based on local regulations.
Can vaccination impact RT-qPCR results?
Vaccination with inactivated vaccines should
not cause PCR positivity because no viral replication occurs. In very rare
instances, very late RT-qPCR Ct values (e.g., >38) may be observed 6–10 days
post-vaccination1. In contrast, naturally infected animals typically
remain viremic for 30–45 days on average (with 0.001% to 8% of cases lasting
more than 60 days4), and BTV RNA can be detectable by PCR for up to
6–7 months5. However, since vaccination usually takes place in
regions where the virus circulates, PCR positivity after vaccination is most
likely due to an infection occurring shortly after vaccination—before full
immune protection is achieved. Therefore, it is essential to carefully analyze
the vaccination status of animals when interpreting RT-qPCR results. Despite
its limitations in determining the precise timing of infection, PCR remains the
only serotype-specific method available for detecting Bluetongue Virus, which
is critical for international animal trade.
References
- WOAH Terrestrial Manual, Chapter 3.1.3. Bluetongue (infection with Bluetongue virus), B. Diagnostics techniques
- Bergh, C., Coetzee, P., & Venter, E. (2018). Reassortment of bluetongue virus vaccine serotypes in cattle. Journal of the South African Veterinary Association, 11, doi:10.4102/jsava.v89i0.1649
- Fay PC et al. (2021). Serological Cross-Reactions between Expressed VP2 Proteins from Different Bluetongue Virus Serotypes. Viruses, 13(8):1455, doi:10.3390/v13081455
- Estimations of the Infective Period for Bluetongue in Cattle, Report of the Scientific Committee on Animal Health and Animal Welfare, Sanco/B3/AH/R12/1999, European Commission Health & Consumer Protection Directorate-General
- Di Gialleonardo L, et al. (2011). The length of BTV-8 viraemia in cattle according to infection doses and diagnostic techniques. Research in Veterinary Science, 91(2):316-20, doi:10.1016/j.rvsc.2010.12.017
- Mann et al., J. Gen. Virol. (2007), 88, 621–630 (1990, adapted from Erasmus, 1990)
For further details, please refer to the original article published in the February 2025 issue of the Boehringer Ingelheim Veterinary Public Health Scientific newsletter on Transboundary Animal Diseases.



 Back
Back